Details of the Drug
General Information of Drug (ID: DMSEIUP)
| Drug Name |
2-iodo-melatonin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
2-Iodomelatonin; 93515-00-5; n-[2-(2-iodo-5-methoxy-1h-indol-3-yl)ethyl]acetamide; CHEMBL289233; N-(2-(2-Iodo-5-methoxy-1H-indol-3-yl)ethyl)acetamide; acetamide,n-[2-(2-iodo-5-methoxy-1h-indol-3-yl)ethyl]-; Acetamide, N-(2-(2-iodo-5-methoxy-1H-indol-3-yl)ethyl)-, (3beta)-; Acetamide, N-(2-(2-iodo-5-methoxy-1H-indol-3-yl)ethyl)-; SR-01000075935; 2-I-MLT; Melatonin,2-Iodo; ML2; Tocris-0737; Lopac-I-1899; AC1L3GZ8; AC1Q5P6Z; Lopac0_000610; SCHEMBL163451; GTPL1343; BDBM29611; CTK8F4316; DTXSID30239462; 2-iodomelatonin; IODOMELATONIN
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
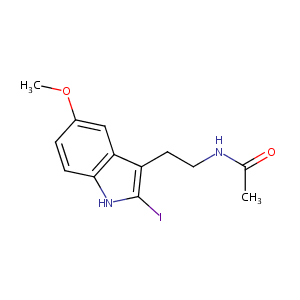 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 358.17 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 2 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||||||||||||
References
